3CLpro Library
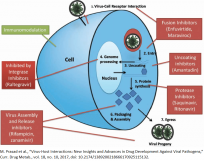
Coronaviruses are single-stranded positive-sense RNA viruses that possess large viral RNA genomes. Usually, beta-coronaviruses produce a ∼800 kDa polypeptide upon transcription of the genome. This polypeptide is proteolytically cleaved to generate various proteins. The proteolytic processing is mediated by PLpro and 3-chymotrypsin-like protease (3CLpro). The 3CLpro, which is also called the ‘main protease’, processes cleave the polyprotein at 11 distinct sites to generate various non-structural proteins that are important for viral replication. Coronaviral 3CLpros are chymotrypsin-like proteases except that they use cysteine as the nucleophile in a catalytic dyad instead of serine in a catalytic triad. 3CLpro exists in a monomer–dimer equilibrium in solution. Each monomer consists of three structural domains: domains I and II contain the catalytic site and chymotrypsin-like scaffold and are connected to a third C-terminal domain via a long loop.
3CLpro plays a critical role in the replication of virus particles and unlike structural/accessory protein-encoding genes, it is located at the 3′ end which exhibits excessive variability. The essential function and conservation among 3CLpros from different coronaviruses make the main protease an attractive drug target for currently known and future emerging coronaviruses. Some natural compounds and their derivatives that possess anti-inflammatory and anti-virus effects exhibit high binding affinity to 3CLpro. [1,2].
Our 3CLPro library is a subset of our SaRS-CoV (2) Targeted Library
The detailed methodology and literature references have been summarized in the corresponding PowerPoint SaRS-CoV (2) Targeted Library slides deck. Specifically, 3CLPro set has been selected using 3D shape similarity virtual screening using APF algorithm.
For the major portion of the 3CLPro subset we were selecting compounds using the ligand from 4TWW PDB X-ray structure as the 3D APF template. The virtual screening (i.e. APF alignment) has been performed setting the protein (i.e. 4TWW PDB X-ray structure) as an excluded volume. For a smaller portion of the 3CLPro subset we have utilized as 3D APF templates a series of designer molecules in collaboration with Insilico Medicine.
[1] M. Tahir ul Qamar, S. M. Alqahtani, M. A. Alamri, and L. L. Chen, “Structural basis of SARS-CoV-2 3CLpro and anti-COVID-19 drug discovery from medicinal plants,” J. Pharm. Anal., Mar. 2020, doi: 10.1016/j.jpha.2020.03.009.
[2] D. Needle, G. T. Lountos, and D. S. Waugh, “Structures of the Middle East respiratory syndrome coronavirus 3C-like protease reveal insights into substrate specificity,” Acta Crystallogr. Sect. D Biol. Crystallogr., vol. 71, pp. 1102–1111, 2015, doi: 10.1107/S1399004715003521.